

Higher and foundation tiers
Separating out mixtures is a task all good chemists need to be able to do well. There are different methods used to separate mixtures, these include: filtration, distillation, chromatography and finally evaporation and crystallisation. The method used depends upon the mixture to be separated. Once you know how each method works then you can decide which one is the best to use for any particular mixture.
A solution is formed when a solute
dissolves in a solvent. As an example consider sugar dissolving in
water to form a sugar solution.
Here the sugar is the solute and the water
is the solvent. A simple particle picture is shown below to offer a simple explanation as to why
some substances dissolve and others do not.
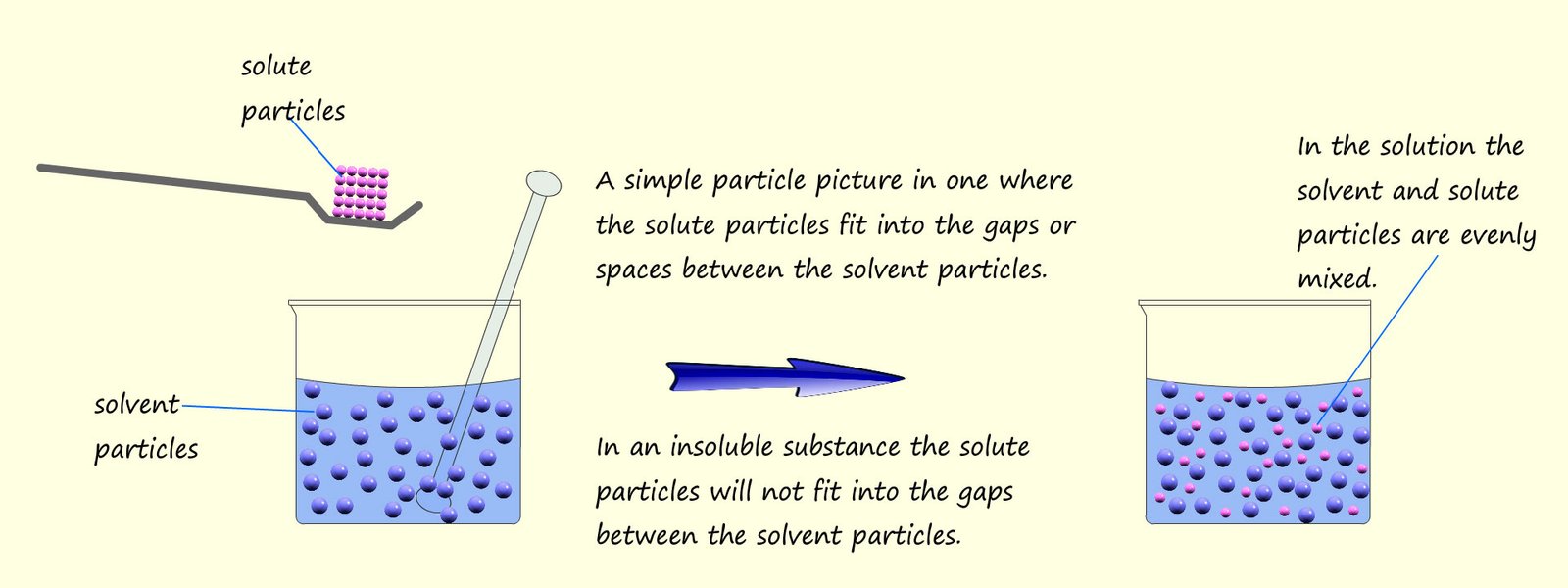
Insoluble substances such as sand or chalk will not dissolve in water. The sand and chalk particles are too large to fit into the gaps between the water or solvent molecules and so are insoluble. It is very easy to separate out an insoluble substance from water (or any other solvent) simply by filtering. The water molecules and any dissolved particles are small enough to pass through the small holes in the filter paper but the sand or chalk molecules are too large to fit through the small holes, so they get trapped in the filter paper. The solid part of any mixture that is trapped in the filter paper is called the residue and the portion that passes through the filter paper is called the filtrate. A simple filtration practical is set out in the image below. Here a mixture of insoluble sand is mixed with a salt water solution.

The filtrate; salt water is a solution of water with salt dissolved in it. How can you separate this salt solution out into salt and water? We cannot filter; filtering only separates insoluble substances from a liquid (a solvent). The simplest way would simply be to pour the salt solution; the filtrate into an evaporating basin and leave it on a warm window ledge for a few days. The water (the solvent) will then evaporate and crystals of salt will start to grow in the evaporating basin. This process of evaporation can be "speeded up" by placing the evaporating basin on a tripod stand and warming gently with a low heat Bunsen burner flame as outlined in the image below:
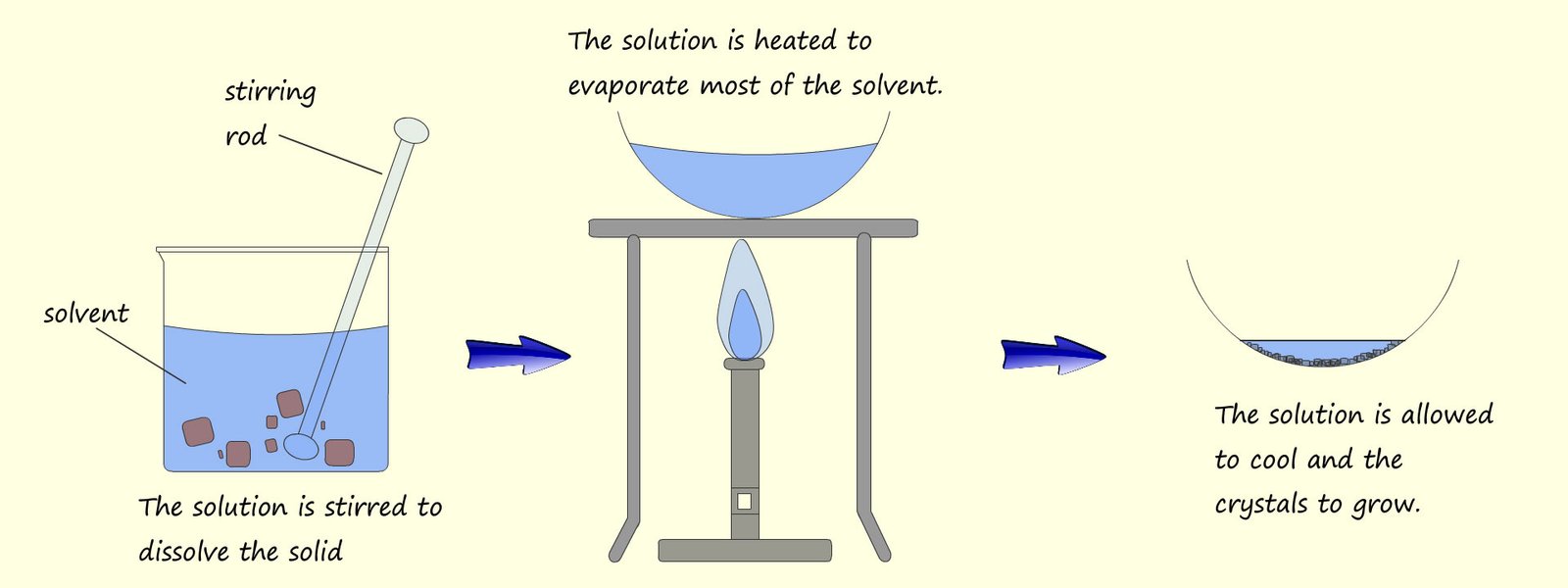
The water (solvent) will start to evaporate and the remaining solution will slowly become more and more concentrated. If most of the solvent is evaporated and the solution is left to cool this will allow time for the crystals to grow on the edges of the evaporating basin. Generally the slower the evaporation occurs the more time the crystals will have to grow and the larger the crystals become. The image below shows the set-up you may have used in class to obtain solid crystals by evaporation.

 We can summarise crystillisation as happening in a series of steps:
We can summarise crystillisation as happening in a series of steps:
Match the terms below with the correct defintions by simply clicking on the term and then its correct defintion. Correct responses will turn the defintions green!